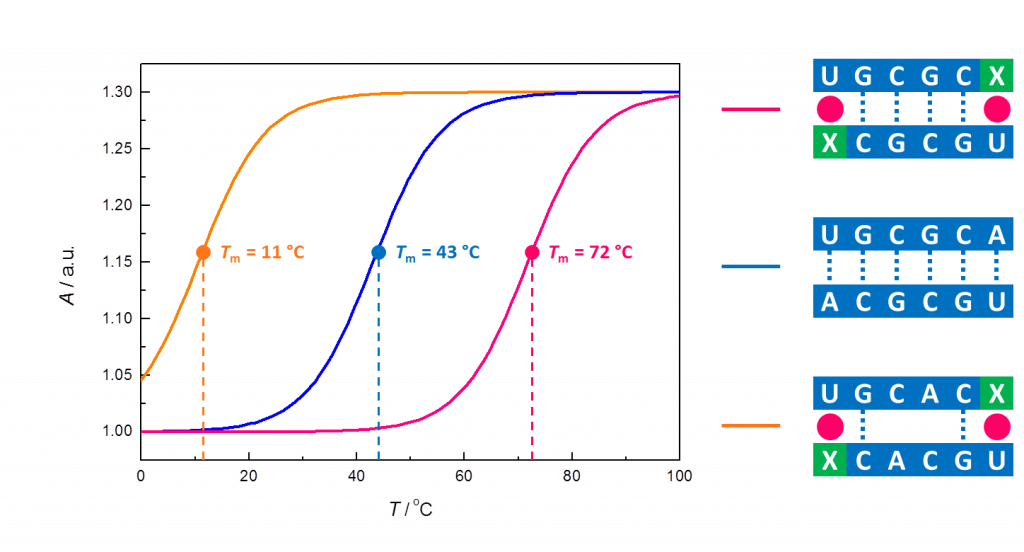
Metal-ion-mediated base-pairing of nucleic acids is attracting interest, owing to the desire to expand the genetic code by artificial base-pairs, to create a predesigned molecular architecture by metal-ion mediated interstrand cross-links, and to impregnate DNA with metal ions for nanotechnological applications. Our interest, however, is recognition of natural nucleic acid sequences by base-modified oligonucleotides that tightly bind metal ions.
The desired high affinity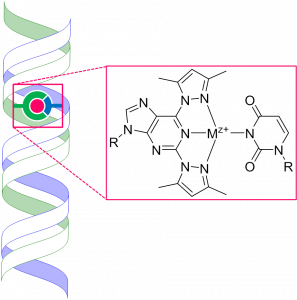 could, in principle, be achieved by coordination of a soft metal ion, such as Pd2+, carried by an artificial nucleobase, to a ring nitrogen (N1 of purines or N3 of pyrimidines) of a natural nucleobase, but discrimination between the four natural nucleobases by metal ion binding surrogate bases on the opposite strand still is an unsolved problem. Tentatively, high affinity binding might be achieved by proper combination of destabilizing steric and stabilizing H-bonding interactions taking place in addition to the metal ion coordination. Some recent observations lend evidence for the feasibility of this kind of an approach.
could, in principle, be achieved by coordination of a soft metal ion, such as Pd2+, carried by an artificial nucleobase, to a ring nitrogen (N1 of purines or N3 of pyrimidines) of a natural nucleobase, but discrimination between the four natural nucleobases by metal ion binding surrogate bases on the opposite strand still is an unsolved problem. Tentatively, high affinity binding might be achieved by proper combination of destabilizing steric and stabilizing H-bonding interactions taking place in addition to the metal ion coordination. Some recent observations lend evidence for the feasibility of this kind of an approach.